Factsheets
Product Testing Fact Sheet
It is important to test your food product to make sure that it will be safe to eat once it is purchased by your customers. Foods that do not meet certain specifications can encourage the growth of bacteria which can cause people to get sick (foodborne illness). A law by the Food and Drug Administration (FDA) called Title 21 provides specifications for different food products to minimize bacterial growth. The SC Department of Agriculture (SCDA) is contracted by FDA to ensure the safety of SC foods. This fact sheet provides important information about the Title 21 requirements and gives detailed instructions for getting your product tested through Clemson University.
Objective of Product Testing
- To make sure consumers get a safe food product that does not encourage the growth of bacteria, leading to foodborne illness.
- To protect you and your business: If someone gets sick after eating your product you can be held responsible (a lawsuit could be brought against you).
Description of What is Being Analyzed During Product Testing at Clemson University
- Acidity and water activity provide information about the potential for bacteria to grow in food. The more acidic the food (low pH) and the less water available in the food (low water activity), the less likely the food will promote bacterial growth. Federal and state food regulations state that a shelf-stable product that does not require refrigeration must have a water activity ≤ 0.85 and a pH ≤ 4.6. Sushi rice pH < 4.2.
- Acidity – measurement of the pH of the food. (pH ranges of some common foods)
- pH: ≤ 4.6 – "acidic food" (Prevents the growth of harmful bacteria)
- pH: ~7.0 – neutral
- pH: ≥8.0 – alkaline
- Water Activity – amount of "free" water (water available to grow bacteria) – Goal is ≤0.85. (Examples of the amount of water activity found in common foods)
- Acidity – measurement of the pH of the food. (pH ranges of some common foods)
- Nutrient Analysis – nutritional content of the food product's ingredients and quantity are analyzed using a food ingredient database which will then generate a nutrition label with all the necessary information in the correct format. We will send you the label in a format that you can take to a printer and use on your product.
Submitting a Food Product for Testing
Print and complete the "Product Testing and Nutritional Labeling Request Form" available on the Food2Market product testing page.
No substitutions for this form will be accepted. This form must be completed in its entirety and submitted with product samples and payment. Failure to submit this completed form will delay product testing results.
Mail the completed "Product Testing and Nutrition Labeling Request Form" along with one sample from four different batches (4 samples total per product) and check** made payable to Clemson University" to:
Clemson University
c/o Dr. Julie Northcutt; Product Testing Laboratory
Department of Food, Nutrition and Packaging Science
223 Poole Agricultural Center
P.O. Box 340316
Clemson, SC 29634-0316
- Note: UPS, post office, and/or FedEx sometimes question this address. Please tell them to send the package to the address above exactly as listed. It will get to the correct location by using this address.
- Packages must include Dr. Julie Northcutt's name as listed above. If not included, we cannot ensure that samples will be tested in a timely manner.
- Products must be mailed to the address listed above. Absolutely no in-person deliveries of product samples will be accepted.
- Broken, leaking, or improperly sealed and marked samples will not be tested.
- If you are sending any products that are perishable, refrigerated or frozen please mark on the outside of the package if the box needs to be refrigerated or frozen upon arrival. Also, email Adair Hoover (cpope@clemson.edu) to expect a refrigerated/frozen package.
- Testing four batches allows us to demonstrate to SCDA that the product is consistent from one batch to the next. Examples of a sample: 1 sample = 1 cup salsa, BBQ sauce, etc., or 1 item such as 1 muffin, 1 piece of candy, etc.
- Sushi rice testing requires samples of RICE from four batches and should be mailed refrigerated. For detailed instructions refer to the Sushi Rice Testing Fact Sheet and Request Form.
- Physical samples are not needed for nutrient analysis (nutrition facts panel; nutrition label) unless you are requesting that we weigh your product to determine the gram weight of a serving size. Otherwise, samples are required for pH and water activity testing only.
- Cash cannot be accepted for payment of product testing. Only checks made payable to "Clemson University" can be accepted at this time.
- Please note that products cannot be accepted when the Product Testing Laboratory is closed. Make sure that samples are not scheduled to be delivered on holidays or weekends. Note that samples received on or after November 24 will not be tested until the lab re-opens in January.)
- For questions about the product testing process please contact Adair Hoover at cpope@clemson.edu or 864-986-4313. Do not call Clemson University's Department of Food, Nutrition and Packaging Science. This office is unable to answer any questions regarding product testing.
- The Product Testing Laboratory is not responsible for lost, spoiled, or broken samples.
- We now accept major national credit cards! You may indicate on your Product Testing Form (or your Sushi Rice Testing Form) that you would like to pay with a credit card. Our office will contact you via telephone during business hours for a credit card number. There is a 3% convenience fee for this service.
Test Results
- Please allow a minimum of four (4) weeks for testing results to be returned.
- Please keep in mind that Clemson University is an educational institution and all faculty, staff, and employees have other responsibilities outside of working with the product testing lab.
- A copy of your results will be sent to you and the SCDA (or other applicable regulatory authority) via e-mail by default or by mail if e-mail is not available. Maintain a copy of these results for your records as the SCDA (or other applicable regulatory authority) can audit your process at any time and you will be held liable if you do not have evidence of your product testing.
Interpreting Your Results
- Products classified as an acidic food (pH ≤ 4.6) can continue to be produced and marketed within an inspected and approved facility.
- A co-packer is a food processing plant that will produce and package your food product for you (AKA co-packers) List of Co-Packers
- You may rent space from a co-packer or shared kitchen in order to make your product or you can hire workers at the co-packer to make the product for you.
- Production of food products in home kitchens is not permitted.
- If your food product is not classified as 'acidic', you will receive recommendations on how to proceed which may include, but are not limited to, product heating, use of sterilized bottles for packaging, and attendance at a Better Process Control School. You will also be required to schedule your process with the FDA.
References
Parisi1, M. A., E. L. Steinberg2, and J. K. Northcutt. 2012. Product testing and nutrition labeling factsheets. Prepared for the Department of Food, Nutrition and Packaging Sciences, Clemson University. 1Assistant Professor, Winthrop University, Rock Hill, SC and Adjunct Assistant Professor, Clemson University; 2Graduate Research Assistant, Clemson University; 3Professor, Clemson University.
Nutrition Labeling Fact Sheet
The 1990 Nutrition Labeling and Education Act was established to provide consumers with accurate information about what is in the food products they are eating. The law provides rules for nutrition labeling that must be followed for all multi-ingredient foods. In 2016, a new Nutrition Facts label was made by the FDA. Clemson can analyze the nutritional content of your food product using a food ingredient database which will then generate a nutrition label with all the necessary information in the correct format. We will send you the label in a format that you can take to a printer and use on your product.
Objective of nutrition labeling
A nutrition label provides information on the ingredients and nutritional make-up of a food including the amount of calories, carbohydrate, fat, protein, and a limited number of vitamins and minerals that the food contains.
It also provides valuable information for people following specific diet guidelines such as those required for diabetes, heart disease, and high blood pressure. The new label introduced in 2016 was designed to help consumers make better food choices for their health, and is based on the most recent scientific data.
Components of a nutrition label
There are three major parts to a nutrition label: the product name including health claims, the ingredient list, and the nutrition facts panel.
The product name and any health claims are present on the front of the packaging. Health claims are closely regulated by the FDA (Food and Drug Administration) and must meet certain criteria. For example, a food that is labeled "low fat" must not have more than 3 grams of total fat per serving. A food labeled "reduced fat", however, has different criteria. It is illegal to use these health claims without first ensuring your product meets these specifications.
The ingredient list is a listing of all of the ingredients in the recipe of your food product. Ingredients are listed in order of weight with the ingredient present in the most amount/weight listed first.
The nutrition facts panel provides detailed information about the nutritional make-up of the food product. The nutrient amounts listed are based on the consumption of one serving of the food product, making "serving size" a key factor in the interpretation of the information.
Changes to the nutrition facts label
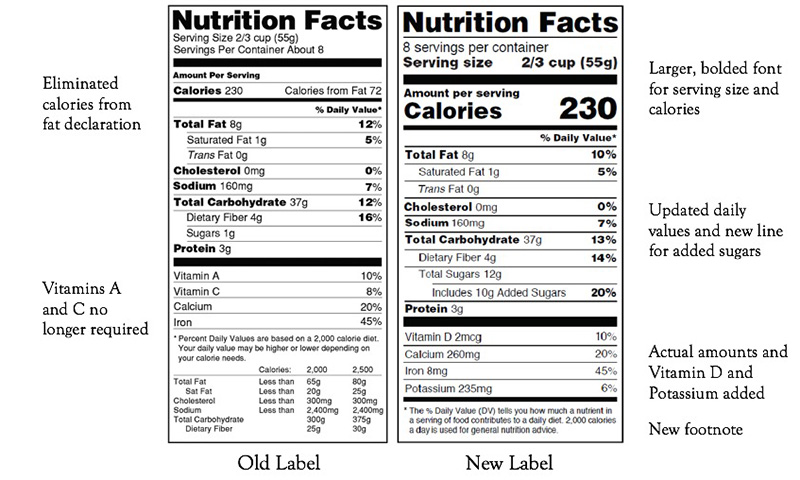
There are many important changes to the new Nutrition Facts panel that was released in 2016. The label remains the same visually for the most part, but many of the details have been changed.
One of the first changes you will notice is the increase in the font size of calories, servings per container, and serving size. Along with the increase in font size, the font has been bolded for the declaration of Calories and serving size. The footnote has also changed to provide a better explanation of the concept of "Daily Value."
Along with the overall visual changes, there are many changes to the breakdown of the nutrient composition section of the label. A new line under the Total Carbohydrate heading was added to include the declaration of added sugars. The calories from fat information have been removed from the label altogether. The vitamin and mineral information has been updated to require the actual amounts of each of vitamin D, potassium, calcium, and iron. The old version of the label required vitamins A and C to be on the label, but they are no longer required. Nutrients that are not required on the label can be included if desired. Using new scientific evidence, the Daily Values have been tweaked to better represent the new Dietary Guidelines for Americans that was published in 2015.
Manufacturers are now required to declare serving sizes based on what a person would actually eat in one sitting. As an example, ice cream now has a referenced serving size of 2/3 cup instead of its previous reference of ½ cup. Some specific products that can be eaten in one sitting or in multiple sittings will require a dual column label representing both the nutrition of the product if eaten in one sitting and the nutrition of the product per intended serving.
Compliance for the new label
The new label is required for manufacturers that have $10 million or more annual food sales by July 26, 2018. Those with annual food sales of less than $10 million will have until July 26 of 2019 to adopt the new label.
Comparing old label vs new label
Requesting Nutrition Labeling
If you have requested product testing from Clemson University by completing the Product Testing and Nutrition Labeling Form , we have the information needed to generate a food label for you. You must make sure to check the 'Nutrition Label' box and complete the form in its entirety in order to have the nutritional analysis completed. The cost is $100.
Mail the completed Product Testing and Nutrition Labeling Form and check** made payable to Clemson University to:
Clemson University
c/o Dr. Julie Northcutt; Product Testing Laboratory
Department of Food, Nutrition and Packaging Science
223 Poole Agricultural Center
P.O. Box 340316
Clemson, SC 29634-0316
- *Note: UPS, post office, and/or FedEx sometimes question this address. Please tell them to send the package to the address above exactly as listed. It will get to the correct location by using this address.
- *Packages must include Dr. Julie Northcutt's name as listed above. If not included, we cannot ensure that samples will be tested in a timely manner.
- Allow four (4) weeks for analysis to be completed and the label to be returned.
- Your label will be sent to you via e-mail by default or to the address provided on the Product Testing and Nutrition Labeling Form.
- We now accept major national credit cards! You may indicate on your Product Testing Form (or your Sushi Rice Testing Form) that you would like to pay with a credit card. Our office will contact you via telephone during business hours for a credit card number. There is a 3% convenience fee for this service.
Interpreting Your Results
It is important to note that Clemson does not actually print labels for you, but we provide the nutrition label to you in a computer file or by email that can be sent to a printer. The file will be saved as an Adobe PDF which is typically the format requested by print shops.
You are advised not to make specific health claims on your food product until you have consulted with officials from the FDA to ensure your product meets the necessary standards
Reference
Food and Drug Administration (FDA). 2017. Changes to the nutrition facts label. Silver Spring MD: U.S. Food and Drug Administration.
Reviewed and updated by Andrew McCullough, Food Science Student and HGIC Intern, Clemson University.
Originally prepared by Parisi 1, M. A., E. L. Steinberg 2, and J. K. Northcutt 3. 2012. Product testing and nutrition labeling factsheets. Prepared for the Department of Food, Nutrition and Packaging Sciences, Clemson University. 1Assistant Professor, Winthrop University, Rock Hill, SC and Adjunct Assistant Professor, Clemson University; 2Graduate Research Assistant, Clemson University; 3Professor, Clemson University.
Sushi Rice Testing Fact Sheet
It is important to test your food products for safety. Foods that do not meet certain specifications can encourage the growth of bacteria and cause people to get sick (foodborne illness).
In South Carolina, the Department of Health and Environmental Control (DHEC) regulates food safety in retail food establishments. There are specific DHEC regulations for sushi rice. Normally prepared rice (no added acid) has a pH range of 6.0 – 6.7 which makes it a time-temperature control for safety food (TCS). TCS foods require strict attention to time/temperature control during preparation, holding, serving, and storing. Sushi rice is often prepared in a manner that cannot realistically meet the time and temperature requirements for TCS foods. So, as an alternative, sushi rice can be "acidified" and treated as a non-TCS food. The addition of vinegar is a common method for acidifying sushi rice. Properly acidified sushi rice will have a pH value of below 4.2. DHEC requires that establishments, who prepare rice for sushi, obtain a variance to standard regulations. The variance is approved when sushi rice preparation consistently produces a product with the required pH of less than 4.2. Sushi rice testing with the Clemson University Product Testing Lab is accepted by DHEC for pH documentation.
The following detailed instructions for sushi rice testing at Clemson University will guide you to through the testing process.
Objective of Product Testing
- To make sure consumers get a safe food product that does not encourage the growth of bacteria, leading to foodborne illness.
- To protect you and your business: If someone gets sick after eating your product you can be held responsible and subject to a lawsuit.
- Provide documentation that the process that your establishment uses to produce sushi rice results in an acceptable pH for classification as a non-TCS food.
Description of Product Testing Analysis
- Sushi rice will be tested for acidity. Acidity provides information about the potential for bacteria to grow in sushi rice. The more acidic the food (low pH), the less likely the food will promote bacterial growth. Federal and state food regulations allow sushi rice to be classified as non-TCS when pH is measured below 4.2.
Submitting Sushi Rice for Product Testing
Print and complete the "Sushi Rice Product Testing Request Form" available on the Food2Market Product testing Page. No substitutions for this form will be accepted. This form must be completed in its entirety and submitted with product samples and payment. Failure to submit the completed form will delay product testing results.
Package should include:
- "Sushi Rice Product Testing Request Form"
- One refrigerated sample of sushi RICE from four different batches (4 samples total)
- Check** made payable to "Clemson University"
Mail to:
Clemson University
c/o Dr. Julie Northcutt; Product Testing Laboratory
Department of Food, Nutrition and Packaging Science
223 Poole Agricultural Center
P.O. Box 340316
Clemson, SC 29634-0316
*Note: UPS, post office, and/or FedEx sometimes question this address. Please tell them to send the package to the address above exactly as listed. It will get to the correct location by using this address.
*Packages must include Dr. Julie Northcutt's name as listed above. If not included, we cannot ensure that samples will be tested in a timely manner.
Keep Refrigerated
- Products must be mailed to the address listed above. Absolutely no in-person deliveries of product samples will be accepted.
- Please mark on the outside of the shipping package "refrigerated or frozen upon arrival".
- Ship samples in refrigerated leak-proof containers. Broken, leaking, or improperly sealed and marked samples will not be tested. Some examples of acceptable containers are Tupperware, plastic, and heavy-duty zip lock bags. Samples may be kept refrigerated by surrounding the sample containers with freezer packs.
- Email Adair Hoover (cpope@clemson.edu) to let her know to expect a refrigerated sample.
- Each sushi rice sample should equal approximately 1 cup. Testing four batches allows us to demonstrate to SC DHEC that the product is consistent from one batch to the next.
- Cash is not an acceptable method of payment for product testing. Checks are accepted but must be made payable to "Clemson University".
- Please note that products cannot be accepted when the Product Testing Laboratory is closed. Make sure that samples are not scheduled to be delivered on holidays or weekends. (Note that samples received on or after November 24 will not be tested until the lab re-opens in January.)
- For questions about product testing process please contact Adair Hoover at cpope@clemson.edu or 864-656-9986. Do not call Clemson University's Department of Food, Nutrition and Packaging Science. This office is unable to answer any questions regarding product testing.
- The Product Testing Laboratory is not responsible for lost, spoiled, or broken samples.
- ** We now accept major national credit cards! You may indicate on your Product Testing Form (or your Sushi Rice Testing Form) that you would like to pay with a credit card. Our office will contact you via telephone during business hours for a credit card number. There is a 3% convenience fee for this service. **
Test Results
- Please allow a minimum of four (4) weeks for testing results to be returned
- Please keep in mind that Clemson University is an educational institution and all faculty, staff, and employees have other responsibilities in addition to the product testing lab.
- A copy of your results will be sent to you and a SC DHEC regulatory authority via e-mail by default or by mail if e-mail is not available Maintain a copy of these results for your records as SC DHEC authority can audit your process at any time and you will be held liable if you do not have evidence of your product testing.
Interpreting Your Results
- Sushi rice that has a tested pH value of less than 4.2 will be approved for classification as a non-TCS food.
- A pH value at or above 4.2 will require that you increase acid in rice and submit additional samples. Cost for additional testing is $25
References
Parisi1, M. A., E. L. Steinberg2, and J. K. Northcutt. 2012. Product testing and nutrition labeling factsheets. Prepared for the Department of Food, Nutrition and Packaging Sciences, Clemson University. 1Assistant Professor, Winthrop University, Rock Hill, SC and Adjunct Assistant Professor, Clemson University; 2Graduate Research Assistant, Clemson University; 3Professor, Clemson University.
Juice HACCP Fact Sheet
Raw fruits and vegetables that are squeezed for juice and stored for future service are subject to specific food safety requirements. That is because like other ready to eat foods, there is no cooking step to help with the destruction of potentially hazardous pathogens. When fresh produce is harvested and transported from fields and farms, there exists the possibility that pathogens may not be fully removed during subsequent cleaning steps. Even a very small number of pathogens can multiply to unsafe levels under extended storage.
Foodborne illnesses from raw juices
The use of contaminated produce in the preparation of raw juices has been linked as a common factor in foodborne illness outbreaks. Acid juices have most commonly been implicated. Potentially hazardous pathogens for acidic juices (pH 4.6 or less) include enteric bacterial pathogens, such as E. coli O157:H7, various Salmonella species, and the protozoan parasite Cryptosporidium parvum. These microorganisms inhabit the intestinal tracts of animals and are excreted through manure or feces. When animals are located in an area near crops, produce can become contaminated through direct contact with feces or indirectly through contaminated irrigation water or runoff. Other possible contaminants of acidic juices are organisms that are pervasive in nature, such as Listeria monocytogenes.
Low acid juices have the potential to become unsafe, too. They are capable of harboring harmful microorganisms, so when prepared for raw service, consideration should be given to toxins produced by non-proteolytic and proteolytic strains of Clostridium botulinum as potential hazards to be controlled.
Raw juice regulations
In retail establishments, raw juice prepared for future service is regulated by the South Carolina Department of Health and Environmental Control (SC DHEC). Retail business refers to establishments that prepare food for direct, on-premise sales and service.
Juice is defined as the aqueous liquid expressed or extracted from one or more fruits or vegetables, purees of the edible portions of one or more fruits or vegetables, or any concentrate of such liquid or puree. This publication strictly pertains to juices that are prepared on-site for immediate service and/or stored for future service.
Retail establishments that offer raw juices are required to apply to SC DHEC for a variance and may be required to develop and implement a juice hazard analysis and critical control point (HACCP) safety plan.
Juice variance requirements
- Remain properly refrigerated below 41 °F.
- Be date marked for no more than 7 days holding followed by the discard of any unused juice at the end of hold time.
- Be treated under a HACCP plan to attain a 5-log reduction, which is equal to a 99.999% reduction, of the most resistant microorganisms of public health significance.
- OR
- Labeled, "WARNING: This product has not been pasteurized and, therefore, may contain harmful bacteria that can cause serious illness in children, the elderly, and persons with weakened immune system".
- The warning statement shall appear prominently and conspicuously on the information panel or on the principal display panel of the label of the container.
- The word "WARNING" shall be capitalized and shall appear in bold type.
- The warning statement of this section, when on a label, shall be set off in a box by use of hairlines.
Establishments that are processing (canning or bottling) raw juices with the intent of creating a shelf-stable product and have met FDA requirements for registration and Better Process Control School may not be required to implement a HACCP plan. However, it is strongly encouraged.
What is HACCP?
HACCP is a food safety system that helps to achieve active control of foodborne illness risk factors. Implementing a HACCP plan for raw juices adds a layer of food safety security by focusing on prevention, not reaction.
These plans require that establishments strictly follow seven principals. The seven principals are common to all HACCP plans; however, each establishment will have a unique plan that reflects their products and preparation methods.
The seven HACCP principals:
- Conduct a hazard analysis: This step identifies potential biological, chemical, and physical hazards. Common examples for raw juices include: listeria, salmonella), norovirus, allergens from cross-contamination, and fragments from glass or metal.
- Determine the critical control points (CCPs): The point or place during the flow of food through manufacturing where significant hazards are most likely to occur.
- Establish critical limits: The precise thresholds for minimizing or eliminating hazards that are deemed to be significant.
- Develop monitoring procedures: A strict plan that identifies what, how, who, and how often the critical control points and limits are monitored.
- Identify corrective actions: If something goes wrong, what will be done to correct the problem? This should be very specific and established before production starts.
- Verify that the system works: Observation and documentation to confirm that the HACCP plan is working.
- Establish procedures for record-keeping and documentation: Procedures are planned in advance and include an establishment's specific HACCP plan, operational records, and documentation of monitoring. It should also define where and for how long records will be stored and maintained.
Additional practices
Additional food safety practices that are critical to safely preparing and storing raw juices include a Standard Operating Procedure (SOP) that emphasizes:
- Pathogen reduction of microbes that an establishment identifies as pertinent microorganisms through cleaning or pasteurization*,
- Prevention of cross-contamination,
- Temperature control throughout the flow of service,
- Employee hygiene program,
- Purchasing from an approved source,
- Avoid premixing different types of juices, but rather mix on order.
*Commercial processors for raw juices are required by FDA to obtain a 5-log reduction of the most resistant organism of public health significance for their individual juice product. Pasteurization is one of the most efficient and reliable ways to obtain that 5-log reduction. High-temperature short-time (HTST) pasteurization is a method of heat pasteurization for perishable beverages including fruit and vegetable juices, which compared with other pasteurization processes maintains color and flavor better.
Pasteurization examples
For an apple juice at pH values of 4.0 or less, the FDA recommends the following thermal processing procedures be followed to achieve the desired 5-log reduction for oocyst of Cryptosporidium parvum, as well as the other three previously mentioned vegetative bacterial pathogens, based upon a conservative evaluation of the available scientific data:
- 160 °F for 6 seconds (recommended treatment conditions in New York),
- 165 °F for 2.8 seconds,
- 170 °F for 1.3 seconds,
- 175 °F for 0.6 seconds,
- OR
- 180 °F for 0.3 seconds
Special requirements
There are special requirements for establishments that serve high-risk populations. These include:
- Juices that bear a warning label and have not been processed per 3-404.11 to reduce or eliminate pathogens may not be served or sold.
- Unpackaged juices prepared on-premise for sale or service in ready-to-eat form shall be processed under a HACCP Plan as specified under 8-201.14 (B-E) and 21 CFR 120(B), 120.24 Process Controls.
Sources
- SC DHEC Retail Food Establishments Regulation 61-25 July 2014: (section 3-404.11(B))
- Guidance for Industry: Juice HACCP Hazards and Controls Guidance First Edition; Final Guidance
Prepared by Adair Hoover, Food Safety Extension Agent, Clemson University. (New 01/17.)
Fermentation Guidelines Factsheet
It is crucial that good manufacturing practices including cleanliness and proper hand washing be meticulously followed during all stages of the fermentation process.
These practices include:
- During processing, thoroughly wash fresh produce, the preparer's hands, cutting utensils/boards, and all containers.
- Select vegetables that are sound, undamaged, uniformly sized and at the proper ripeness.
- Fermentation vessel must be located in a secure location during fermentation.
- Culture should not be backslopped.
- The absence of oxygen is required during fermentation. Preparer must cover product with liquid to exclude air during fermentation. Seal the fermentation vessel to exclude oxygen and ensure anaerobic conditions.
- If product becomes discolored (pink or dark) it should be discarded. This discoloration indicates spoilage.
- Detailed records during production of temperatures and pH are required.
- Equipment calibrations are required and should be documented.
- Retain records for 2 years (varies for different products).
- Once fermentation is complete, keep product under refrigeration (less than 40 °F).
- Fermented foods that are processed for shelf stability require product testing by a process authority to establish an adequate thermal process specific to the product.
- Fermented foods are not considered acidified and therefore do not require registration with the FDA to sell.
Fermentation Processes
| Food | Optimum Salt Level | Optimum Temperature Range | Time Range to Complete Fermentation |
|---|---|---|---|
| Cucumbers | 5-8% (Brine) | 59-89.6 °F | |
| Cabbage | 2.25% (by weight of cabbage) | 60-70 °F | *5-6 weeks |
| 70-75 °F | *3-4 weeks | ||
| Kimchi | 4-6% (brine or by weight) | 50-64 °F | *5-20 days |
| Fruit | 2-3% (brine or by weight) | 50-59 °F | 2-6 weeks |
* Fermentation is complete when product reaches a pH below 4.6
| Food | Starter Culture | Optimum Temperature Range | Time Range to Complete Fermentation |
|---|---|---|---|
| Kombucha | Heat to rolling boil before adding | 75-85 °F | 12 days minimum |
Guidelines for Kombucha production:
- If using a dehydrated starter culture, such as SCOBY, the manufacturer's guidelines must be followed for rehydration with vinegar.
- Fermentation may be performed at lower temperatures. If this is done, holding times must be extended to appropriately compensate for reduced microbiological respiration due to lower temperatures.
- The pH must be tested by a Process Authority.
- Must be lab tested to confirm that finished product contains less than 0.5% alcohol by volume.
- Sterile bottles must be used if any bottling occurs.
- Product should be kept refrigerated (below 40 °F) once fermentation is complete to avoid further alcohol production.
- Product should be labeled as "keep refrigerated"
Additional fermented foods may be evaluated on an individual basis.
Prepared by Adair Hoover, HGIC Food Safety Agent, and Kimberly Baker, PhD, RD LD, State Consumer Food Safety Program Coordinator, Clemson University. (New 01/17.)
The Food Safety of Sprouts Fact Sheet
Sprouts are the immature growth that is produced from a germinated seed. Depending on the seed type, the sprout is generally harvested 1 to 8 days after germination. At harvest the sprout will have a stem (1 to 3 inches in length) and two small leaves. The varieties of sprouts that are most commonly consumed are alfalfa, mung bean, red clover, radish, broccoli and wheat grass. Sprouts are most commonly consumed raw or lightly cooked as they provide a crisp texture to sandwiches, salads and stir-fries. Unfortunately, the environment that the sprout needs for growth, combined with the fact that they are generally eaten raw or only lightly cooked, has caused many foodborne illness outbreaks.
Sprouts and Foodborne Illness
Between 1996 and 2010, there were 34 reported foodborne illness outbreaks related to the consumption of sprouts. These outbreaks resulted in 2,150 cases of illness, 123 hospitalizations and one death. In the past 5 years (2011 to 2016), the Centers for Disease Control and Prevention have reported 9 foodborne illness outbreaks that resulted from the consumption of sprouts. Due to the high number of outbreaks, sprouts have been labeled as a "high risk" food. This means that people with compromised immune systems, such as children, elderly, pregnant women and those who are sick or taking medications that impair the immune system, should avoid eating sprouts.
What Causes Sprouts to be "High Risk"?
Sprout seeds can often be the starting point of a foodborne illness outbreak. This is because the seeds can potentially become contaminated during production while growing in the field. In the field, contamination can come from irrigation water, animal manure, wild animals or unsanitary practices or dirty hands of field workers. Upon harvest, seeds can be introduced to contamination from transportation containers and vehicles, equipment, rodents, pests and workers.
Some varieties of seeds naturally have rough outer surfaces that can allow for microorganisms to easily attach. Additionally, some seeds are put through a process called scarification in which the outer surface of the seed is abrasively rubbed to thin the outer seed coat and increase germination rates. This process can also create rough outer surfaces in which microorganisms can hide.
The growing environment of the sprout supports an ideal growing environment for microorganisms. Sprouts require adequate moisture and warm temperatures (about 70°F) both of which create ideal conditions for the rapid growth of microorganisms. One study reported that the number of microorganisms on a sprouting seed can reach up to 1 billion within 2-3 days of the sprouting process. It does not take many cells of microorganisms to cause someone to become sick. For example, someone can consume just one cell of Salmonella or 10 to 100 cells of Escherichia coli (E. coli) and become sick with a foodborne illness.
Safety of Commercially Grown Sprouts
In 2011, the Food Safety Modernization Act (FSMA) was signed into law. This act is a complete overhaul to the United States' food safety system shifting the focus from responding to foodborne illness outbreaks to prevention. Several components to this law are intended to prevent foodborne illness in manufactured foods and produce; however, one aspect of the law specifically addresses the production of sprouts. Those who are producing sprouts for sale must comply with the regulations stated in the FSMA produce safety rule as well as four additional requirements that are specific to growing sprouts. These requirements are: (1) taking steps to prevent microorganisms on seeds; (2) testing irrigation water drained from growing sprouts; (3) testing of the sprout production areas (growing, harvesting, packing and holding) for Listeria monocytogenes; and (4) if any test results in a positive reading then corrective actions must be put into place so that contaminated sprouts are not released for sale. Additional educational trainings are also being given to sprout producers to teach them the new regulations and how to grow sprouts safely.
Growing Sprouts at Home
Growing sprouts at home does not make them any safer than those purchased from the grocery store. Care should be taken when they are grown at home to reduce the likelihood of the sprouts causing a foodborne illness. Seeds should be purchased from a commercial source, where the seeds are produced for sprouting only and pre-tested for the presence of illness causing microorganisms. Ensure that all containers and contact surfaces that touch the seeds and sprouts are kept clean. Place growing sprouts in an area of your home where they are not disturbed and are not located close to food production areas where raw foods can splash onto the sprouts. Keep pets away from the seeds and sprouts. Always wash hands properly when handling the seeds or sprouts, and ensure that the water used to irrigate the sprouts is fresh drinkable water and held in a clean container.
Handling Sprouts at Home
Whether you have purchased sprouts from the store, or grown them at home, you can reduce the chance of developing a foodborne illness from sprouts by following these guidelines:
- Buy/consume only fresh sprouts that are kept refrigerated.
- Do not buy/consume sprouts that are limp, slimy, moldy or have an off odor.
- Keep sprouts refrigerated at 40°F or below.
- Store sprouts in clean containers.
- Wash hands properly with hot running water before touching sprouts.
- Wash sprouts with cool running water directly before use.
- Baker, K.A. 2016. Microbiological and quality characteristics of alfalfa (Medicago sativa) and mung bean (Vigna radiate) sprouts grown using different water sources and treated post-harvest (Doctoral dissertation). Retrieved from ProQuest Dissertations and Theses. (Accession Order No. 1621).
- Centers for Disease Control and Prevention (CDC). 2016. List of selected multistate foodborne outbreak investigations. Atlanta, GA: Centers for Disease Control and Prevention. Accessed December 14, 2016.
- Food and Drug Administration (FDA). 2012. Bad Bug Book, Foodborne Pathogenic Microorganisms and Natural Toxins. Second Edition. Silver Spring, MD: U.S. Food and Drug Administration. Accessed March 24, 2016.
- Food and Drug Administration (FDA). 2015. FSMA final rule on produce safety. Silver Spring, MD: U.S. Food and Drug Administration. Accessed February 27, 2016.
- Johanson, J. 2012. FDA’s strategy of creating alliances. Silver Spring, MD: U.S. Food and Drug Administration. Accessed September 19, 2015.
- Liao, C.H. 2008. Growth of Salmonella on sprouting alfala seeds as affected by the inoculum size, native microbial load and Pseudomonas fluorescens 2-79. Letters in Applied Microbiology 46:232-236.
- Mueller, S. 2008. Alfalfa seed production in the western United States. Fresno, CA: UC Cooperative Extension, University of California Davis. Accessed January 14, 2016.
- Oregon Public Health Division, Belabre, B., Dekevich, D., Dement, J. 2015. Sprouts. Fort Collins, CO: Food Source Information Colorado State University. Available from: www.fsi.colostate.edu/sprouts/. Accessed September 19, 2015.
Prepared by Kimberly Baker PhD, RD, LD, State Consumer Food Safety Program Coordinator, Clemson Extension, Clemson University, and reviewed by Adair Hoover, Food Safety Extension Agent, Clemson Extension, Clemson University (New 12/16)
